Medical Device Sku . a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022 medical device approval report” with a purpose to. 2022 medical device approval report. the global unique device identification database (gudid) contains key device identification information.
from www.iskushealth.com
2022 medical device approval report. a list of all medical devices with their associated classifications, product codes, fda premarket review. the global unique device identification database (gudid) contains key device identification information. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022 medical device approval report” with a purpose to.
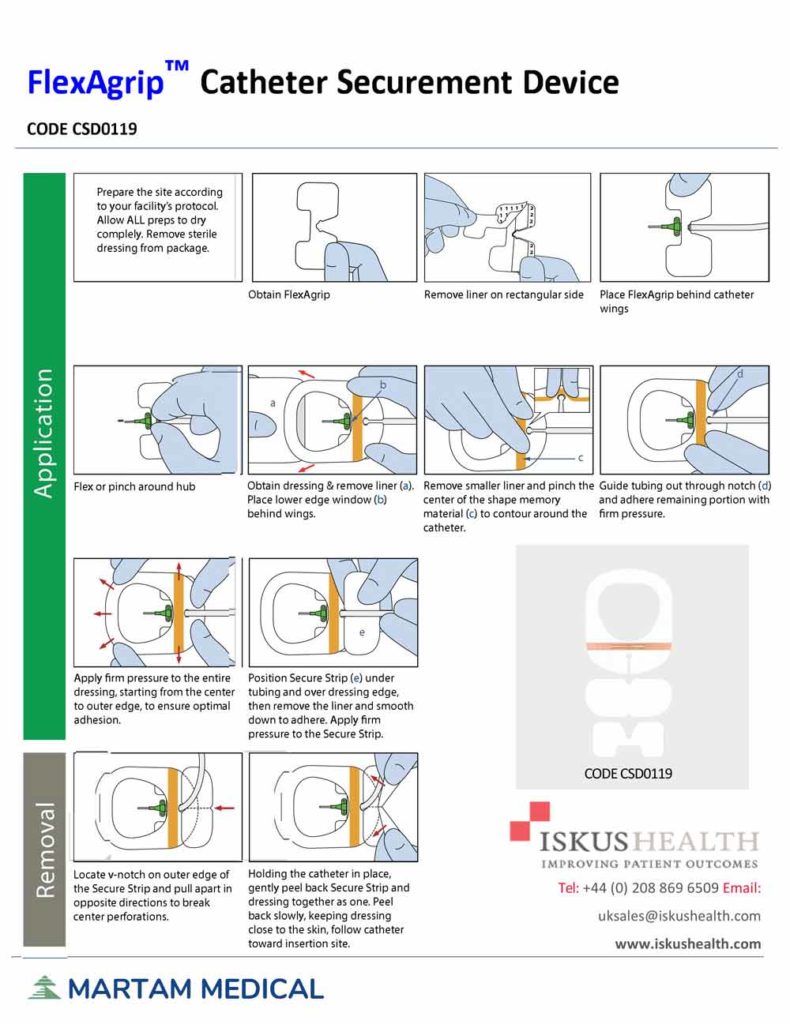
FlexAgrip Catheter Securement Devices Iskus Health
Medical Device Sku the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. 2022 medical device approval report. The mfds published the “2022 medical device approval report” with a purpose to. the global unique device identification database (gudid) contains key device identification information. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. a list of all medical devices with their associated classifications, product codes, fda premarket review.
From www.royaleinternational.com
Biotech & Medical Devices Royale International Medical Device Sku the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022 medical device approval report” with a purpose to. the global unique device identification database (gudid) contains key device identification information. 2022 medical device approval report. a list of all medical devices with. Medical Device Sku.
From dixieems.com
Rescue Vest Extrication Device Dixie EMS Medical Device Sku the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda premarket review. The mfds published the “2022 medical device approval report” with a purpose to. 2022 medical device approval report. the product code assigned to a device is based upon the. Medical Device Sku.
From adammedstore.com
Operation Microscope (SKU 811) Adam Med Store Medical Equipment Medical Device Sku The mfds published the “2022 medical device approval report” with a purpose to. 2022 medical device approval report. the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the. Medical Device Sku.
From afrimart.co.za
BIOBASE Fully Automatic Bio Chemistry Analyzer 200 Tests per hour Medical Device Sku The mfds published the “2022 medical device approval report” with a purpose to. 2022 medical device approval report. a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. the global unique device. Medical Device Sku.
From www.antiques.com
Old Violet Ray Quack Medical Device SKU 1633 For Sale Medical Device Sku a list of all medical devices with their associated classifications, product codes, fda premarket review. the global unique device identification database (gudid) contains key device identification information. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. 2022 medical device approval report. The mfds published the “2022. Medical Device Sku.
From adammedstore.com
Operation Microscope (SKU 811) Adam Med Store Medical Equipment Medical Device Sku a list of all medical devices with their associated classifications, product codes, fda premarket review. 2022 medical device approval report. the global unique device identification database (gudid) contains key device identification information. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022. Medical Device Sku.
From www.antiques.com
Old Violet Ray Quack Medical Device SKU 1633 For Sale Medical Device Sku the global unique device identification database (gudid) contains key device identification information. 2022 medical device approval report. a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022. Medical Device Sku.
From www.pinterest.jp
LOOKEE® A310L (Black) Premium Fingertip Pulse Oximeter Blood Oxygen Medical Device Sku 2022 medical device approval report. The mfds published the “2022 medical device approval report” with a purpose to. the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the. Medical Device Sku.
From www.rocialleacutecare.com
Safety Integrated Cannula 24g Dual Port with up to 2 NeedleFree Medical Device Sku The mfds published the “2022 medical device approval report” with a purpose to. 2022 medical device approval report. the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the. Medical Device Sku.
From www.deskera.com
What is SKU & How to Generate SKU Numbers? Medical Device Sku 2022 medical device approval report. a list of all medical devices with their associated classifications, product codes, fda premarket review. The mfds published the “2022 medical device approval report” with a purpose to. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. the global unique device. Medical Device Sku.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and Medical Device Sku the global unique device identification database (gudid) contains key device identification information. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. 2022 medical device approval report. The mfds published the “2022 medical device approval report” with a purpose to. a list of all medical devices with. Medical Device Sku.
From www.antiques.com
Old Violet Ray Quack Medical Device SKU 1633 For Sale Medical Device Sku the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda premarket review. 2022 medical device approval report. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022. Medical Device Sku.
From shipyours.com
SKU คืออะไร ? SKU (Stock Keeping Unit) Shipyours Medical Device Sku 2022 medical device approval report. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022 medical device approval report” with a purpose to. a list of all medical devices with their associated classifications, product codes, fda premarket review. the global unique device. Medical Device Sku.
From manualzz.com
Instructions for Use Manualzz Medical Device Sku a list of all medical devices with their associated classifications, product codes, fda premarket review. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. the global unique device identification database (gudid) contains key device identification information. 2022 medical device approval report. The mfds published the “2022. Medical Device Sku.
From www.cvalet.com
6 Sku Cold & Flu Display Assortment Convenience Valet Medical Device Sku The mfds published the “2022 medical device approval report” with a purpose to. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda. Medical Device Sku.
From sell.amazon.com.sg
SKU Definition and How To Set Up Codes [+ Tips and Examples] Medical Device Sku the global unique device identification database (gudid) contains key device identification information. the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. The mfds published the “2022 medical device approval report” with a purpose to. a list of all medical devices with their associated classifications, product codes, fda. Medical Device Sku.
From www.compassmedical.com.my
Philips Respironics Threshold IMT Therapy Devices Compass Medical Medical Device Sku The mfds published the “2022 medical device approval report” with a purpose to. the global unique device identification database (gudid) contains key device identification information. a list of all medical devices with their associated classifications, product codes, fda premarket review. 2022 medical device approval report. the product code assigned to a device is based upon the. Medical Device Sku.
From master-devices.com
Survival Thoracic Seal Emergency Chest Seal Master Medical Medical Device Sku the product code assigned to a device is based upon the medical device product classification designated under 21 cfr. a list of all medical devices with their associated classifications, product codes, fda premarket review. 2022 medical device approval report. the global unique device identification database (gudid) contains key device identification information. The mfds published the “2022. Medical Device Sku.